Plum Pudding Model: Important Concept Given in 1904
Table of Contents
Introduction
The plum pudding model, also known as the Thomson model or the raisin pudding model, was a proposed atomic model in the early 20th century. It was put forward by J.J. Thomson in 1904 before the discovery of the atomic nucleus.
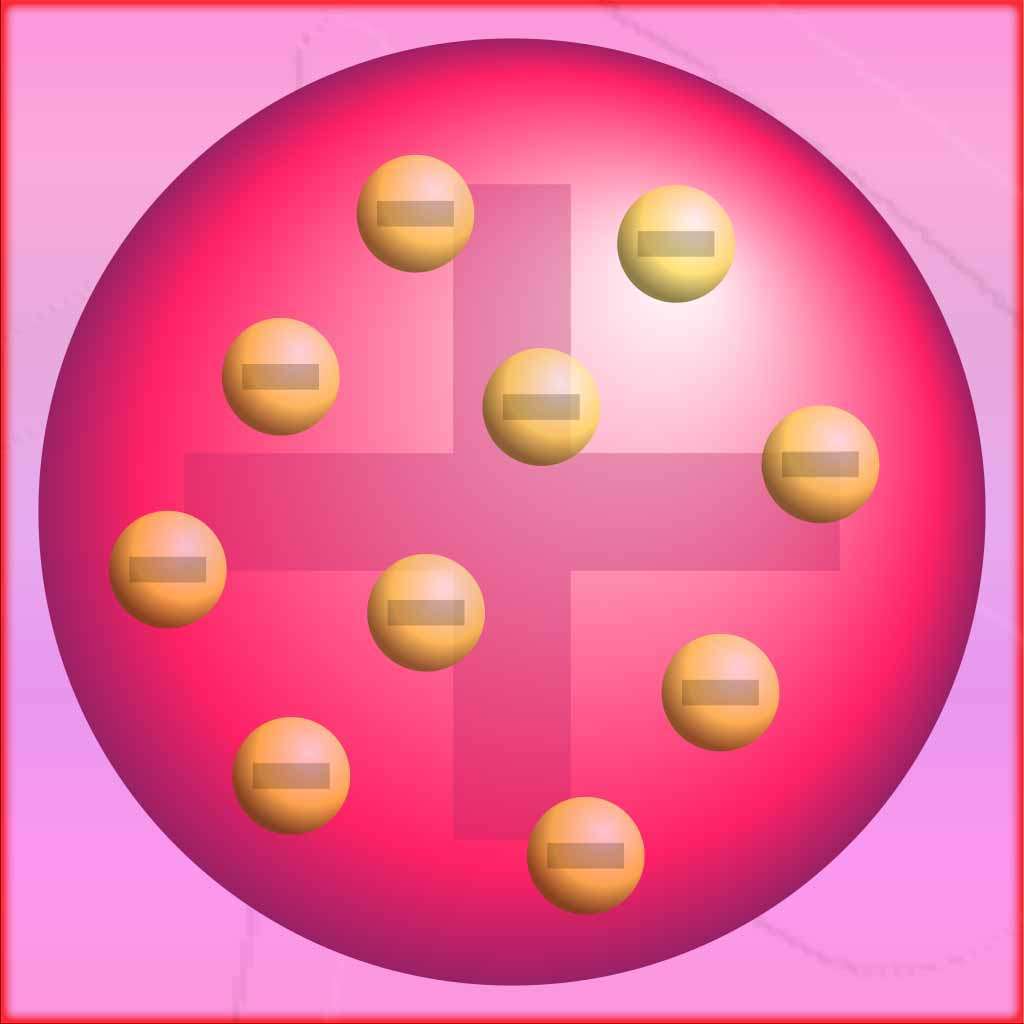
According to the plum pudding, atoms were thought to be composed of a positively charged “pudding” or matrix, in which negatively charged electrons were embedded like “plums.” Thomson’s model was based on his experiments with cathode rays, which showed that atoms contained negatively charged particles.
Thomson’s model suggested that the atom had no internal structure and was a uniform distribution of positive and negative charges. The positive charge was spread throughout the atom, while the negatively charged electrons were interspersed within it. This model was consistent with the observed behavior of cathode rays, which were deflected by electric and magnetic fields.
However, the plum pudding model was eventually replaced by the Rutherford model in 1911 when Ernest Rutherford conducted his famous gold foil experiment. Rutherford’s experiment demonstrated that the atom had a concentrated positively charged nucleus at its center, with electrons orbiting around it.
The plum pudding played a significant role in the development of atomic theory and helped scientists understand the presence of negatively charged particles within the atom. Although it was ultimately superseded by more accurate models, it contributed to the foundation of our understanding of atomic structure.
plum pudding model vs nuclear model
The plum pudding model and the nuclear model are two different atomic models proposed by scientists in the early 20th century.
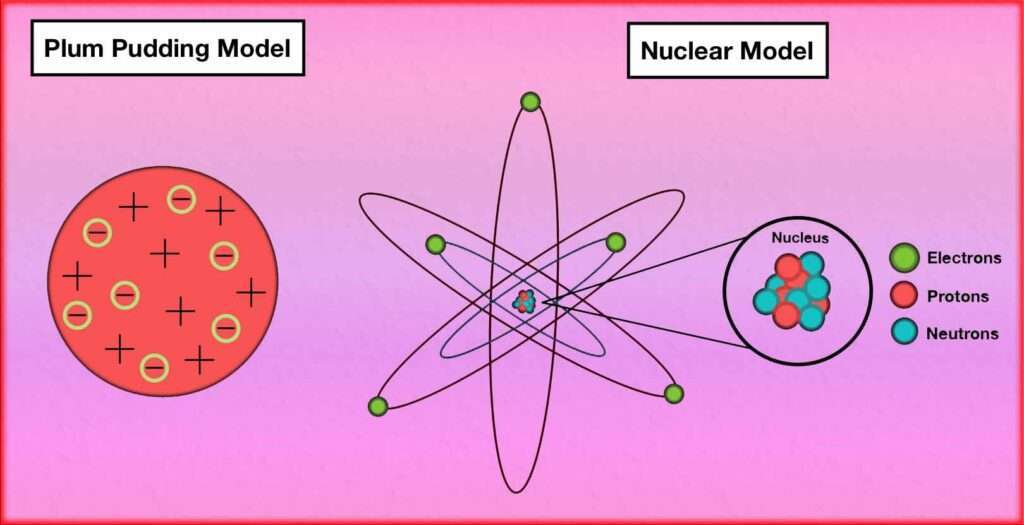
Here’s a comparison between the two:
Plum Pudding Model:
Proposed by J.J. Thomson in 1904.
According to this model, the atom consists of a positively charged “pudding” or matrix with negatively charged electrons embedded in it. The positive charge is spread uniformly throughout the atom.
The model suggests that the atom has no internal structure and is a uniform distribution of positive and negative charges. It was based on Thomson’s experiments with cathode rays and their deflection by electric and magnetic fields.
Nuclear Model:
Proposed by Ernest Rutherford in 1911.
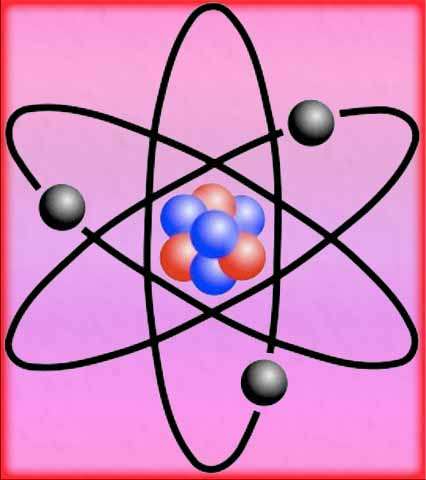
Based on his gold foil experiment, Rutherford discovered that atoms have a small, dense, positively charged nucleus at the center. The nucleus contains most of the atom’s mass and positive charge.
Electrons orbit around the nucleus in specific energy levels or shells. The model suggests that the atom is mostly empty space, with the positively charged nucleus occupying a tiny fraction of the total volume.
comparison of Both Models:
The plum pudding assumes a uniform distribution of positive charge throughout the atom, while the nuclear model acknowledges the concentrated positive charge within the nucleus.
The plum model doesn’t account for the existence of a small, dense nucleus, while the nuclear model recognizes its presence. The pudding Model doesn’t specify the arrangement of electrons, whereas the nuclear model describes electrons orbiting the nucleus in specific energy levels.
The nuclear model superseded the plum model based on the results of Rutherford’s gold foil experiment, which showed that the positive charge is concentrated in a small region.
Overall, the nuclear model provides a more accurate representation of atomic structure by incorporating the concept of a nucleus and the arrangement of electrons in energy levels, whereas the plum pudding model is a simpler model that was later disproven.
why was the plum pudding model replaced
The plum pudding model was replaced by the nuclear model primarily because of the results of the gold foil experiment conducted by Ernest Rutherford in 1911. In this experiment, Rutherford and his colleagues fired alpha particles (positively charged particles) at a thin sheet of gold foil.
According to the model, which proposed a uniform distribution of positive charge throughout the atom, the alpha particles should have passed through the gold foil with only minor deflections. However, the experimental results were unexpected and led to a paradigm shift in atomic theory.
The gold foil experiment showed that while most of the alpha particles passed through the foil as expected, a small fraction of the particles were deflected at large angles and some even bounced straight back. This observation was highly inconsistent with the plum pudding model and raised questions about its validity.
To explain these results, Rutherford proposed the nuclear model, also known as the Rutherford model. According to this model, the atom has a small, dense, and positively charged nucleus at its center, where most of the atom’s mass is concentrated. The electrons orbit around the nucleus in specific energy levels.
The deflections and bounces observed in the gold foil experiment were explained by Rutherford as the result of the positively charged alpha particles coming close to the densely concentrated positive charge of the nucleus. The repulsive forces between the positive charges caused the deflections.
The gold foil experiment provided strong evidence for the existence of a small and massive nucleus within the atom, which directly contradicted the assumptions of the plum pudding model. As a result, the nuclear model replaced the plum pudding as a more accurate representation of atomic structure.
The gold foil experiment and the subsequent development of the nuclear model played a significant role in shaping our understanding of the atom and paved the way for further advancements in atomic theory.
Follow us on LinkedIn“Electrical Insights” to get the latest updates in Electrical Engineering. You can also Follow us LinkedIn to see our latest posts.
Related Topics in Physics
Work Function: Formula Derivation and Threshold Frequency
Period and Frequency: A Comprehensive Analysis
Wavelength And Frequency: 2 Important Parameters
Frequently Asked Questions
What is the plum pudding model?

The plum pudding model is an atomic model that describes the structure of an atom as a positively charged matrix with negatively charged electrons embedded within it.
Who proposed the plum pudding model?
The plum pudding model was proposed by J.J. Thomson.
How does the plum pudding model describe the structure of an atom?
The plum pudding model suggests that the structure of an atom is composed of a positively charged “pudding” or matrix, where negatively charged electrons are dispersed throughout.
What is the significance of the electrons in the plum pudding model?
In the plum pudding model, electrons are significant as they provide the negatively charged component of the atom and are embedded within the positively charged matrix.
How does the plum pudding model explain the behavior of cathode rays?
The plum pudding model explains the behavior of cathode rays by suggesting that these rays are streams of negatively charged electrons emitted from the atoms.
What experimental evidence supported the plum pudding model?
The experimental evidence supporting the plum pudding model primarily came from J.J. Thomson’s experiments with cathode rays, which demonstrated the presence of negatively charged particles within atoms
Why was the plum pudding model eventually replaced?
The plum pudding model was eventually replaced by the nuclear model when the results of the gold foil experiment conducted by Ernest Rutherford showed the existence of a small, dense, and positively charged nucleus at the center of an atom.
What were the limitations of the plum pudding model?
The limitations of the plum pudding model include its failure to account for the concentrated positive charge within the nucleus and its inability to explain the observation of alpha particles being deflected at large angles in the gold foil experiment.
How does the plum pudding model compare to the nuclear model?
In contrast to the plum pudding model, the nuclear model recognizes the presence of a small and dense nucleus at the center of an atom, while electrons orbit around the nucleus in specific energy levels.
What impact did the plum pudding model have on the development of atomic theory?
The plum pudding model played a significant role in the development of atomic theory by contributing to our understanding of the presence and behavior of electrons within atoms. It paved the way for further investigations and the eventual formulation of more accurate atomic models.
