Work Function in Photoelectric Effect: Best Guide
Understanding Work Function in Photoelectric Effect
The work function in photoelectric effect is a fundamental concept in physics that explains how light interacts with metals to eject electrons. It represents the minimum energy required to remove an electron from the surface of a material. This principle is crucial in understanding various applications, including solar cells, photodetectors, and vacuum tubes.
The photoelectric effect was first explained by Albert Einstein in 1905, building upon Max Planck’s quantum theory. It provided strong evidence for the particle nature of light, leading to the development of quantum mechanics. The work function plays a central role in this phenomenon, as only photons with energy equal to or greater than this threshold can cause electron emission.
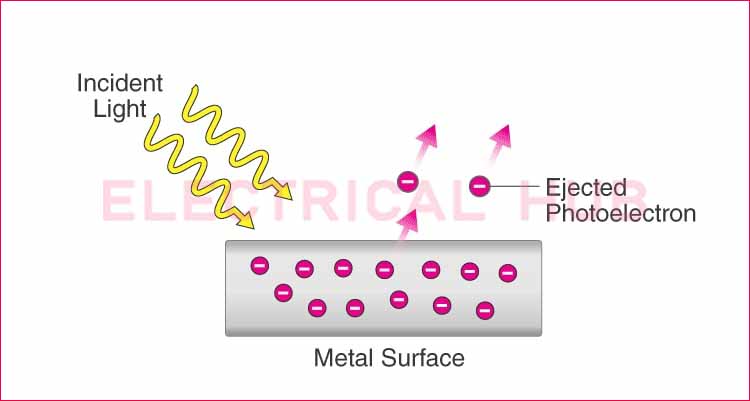
Table of Contents
Table of Contents
Work Function Definition
The work function of a material is the least amount of energy required to remove a free electron from its surface. It is typically measured in electron volts (eV) and depends on various factors, including the material’s composition and surface conditions.
For metals, the work function varies widely. For example:
- Cesium – 2.1 eV (Low work function, easily emits electrons)
- Copper – 4.7 eV (Higher work function, harder to eject electrons)
- Platinum – 5.65 eV (Very high work function)
The relationship of work function in photoelectric effect is expressed using Einstein’s equation:
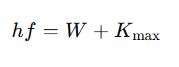
Where:
- h= Planck’s constant (6.626 × 10⁻³⁴ J·s)
- f= Frequency of the incident light
- W= Work function of the material
- Kmax= Maximum kinetic energy of the emitted electron
This equation states that the energy of an incoming photon must at least match the work function to liberate an electron. Any excess energy is converted into the electron’s kinetic energy.
Work Function Formula
The work function formula is mathematically represented as:
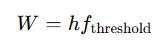
Where:
- W= Work function
- fthreshold= Threshold frequency of the metal
The threshold frequency is the minimum frequency of light needed to eject electrons. If the incident photon has a frequency lower than this threshold, no electrons will be emitted, regardless of the light intensity.
Factors Work Function Depends On
The work function depends on multiple factors, including:
Material Type – Different metals and compounds have unique work functions due to their atomic structures.
Surface Purity – Contaminants on the metal surface can alter the work function by either increasing or decreasing the required energy.
Temperature – Increasing temperature can reduce the work function slightly due to thermal agitation.
Crystal Structure – Different crystallographic orientations of the same metal can exhibit slight variations in work function.
External Fields – The presence of an electric or magnetic field can modify the work function by influencing the potential barrier at the surface.
Work Function Symbol and Unit
The work function symbol is commonly represented as W or ϕ in equations.
Its standard unit is the electron volt (eV), but in SI units, it is expressed in joules (J). The conversion between these units is:
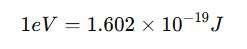
For example, a work function of 4.5 eV is equivalent to:
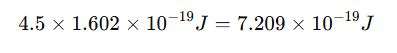
Practical Applications of Work Function in Photoelectric Effect
The concept of work function has significant applications across various fields:
Solar Cells – Photovoltaic cells operate based on the photoelectric effect, where sunlight causes electron emission in semiconductor materials.
Photodetectors – Devices like photodiodes and photomultipliers rely on work function principles to detect light intensity.
Electron Microscopes – Work function influences electron emission in scanning electron microscopes (SEMs), impacting resolution.
Vacuum Tubes – Early electronic devices, such as cathode-ray tubes (CRTs), used thermionic emission, which is closely related to work function.
Photoemissive Coatings – Materials with low work functions are used in light-sensitive coatings for optical sensors and detectors.
Experimental Measurement of Work Function
To determine the work function of a material, several methods are used:
1. Photoelectric Method
- A monochromatic light source illuminates a clean metal surface in a vacuum.
- The emitted electrons’ kinetic energy is measured, and the work function is calculated using Einstein’s equation.
2. Kelvin Probe Method
- A non-contact method that measures the difference in work function between a reference electrode and the test material.
3. Thermionic Emission Method
- Measures the electron emission rate from a heated surface, using the Richardson-Dushman equation:
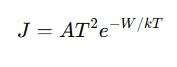
Where:
- J= Current density
- A= Richardson constant
- T= Absolute temperature
- k= Boltzmann constant
Conclusion
The work function in photoelectric effect is a crucial parameter that defines the minimum energy needed for electron emission. It plays a fundamental role in modern physics and technology, especially in areas like solar energy, photodetection, and vacuum electronics.
Understanding the work function formula, work function definition, and the factors work function depends on provides a comprehensive insight into its significance. Since the work function unit is often given in electron volts, precise calculations are necessary for practical applications.
By leveraging materials with appropriate work function symbols and values, engineers and scientists can optimize devices for improved efficiency and performance in various technological applications.
Follow Us on Social:
Subscribe our Newsletter on Electrical Insights for latest updates from Electrical Engineering Hub
WorkFunction #PhotoelectricEffect #PhysicsConcepts #QuantumMechanics #ElectronEmission #EnergyThreshold #PhysicsExplained #WorkFunctionFormula #LightEnergy #MetalSurfaces #Photoemission #PhysicsLaws #WorkFunctionUnit #ElectromagneticRadiation #ScientificPrinciples