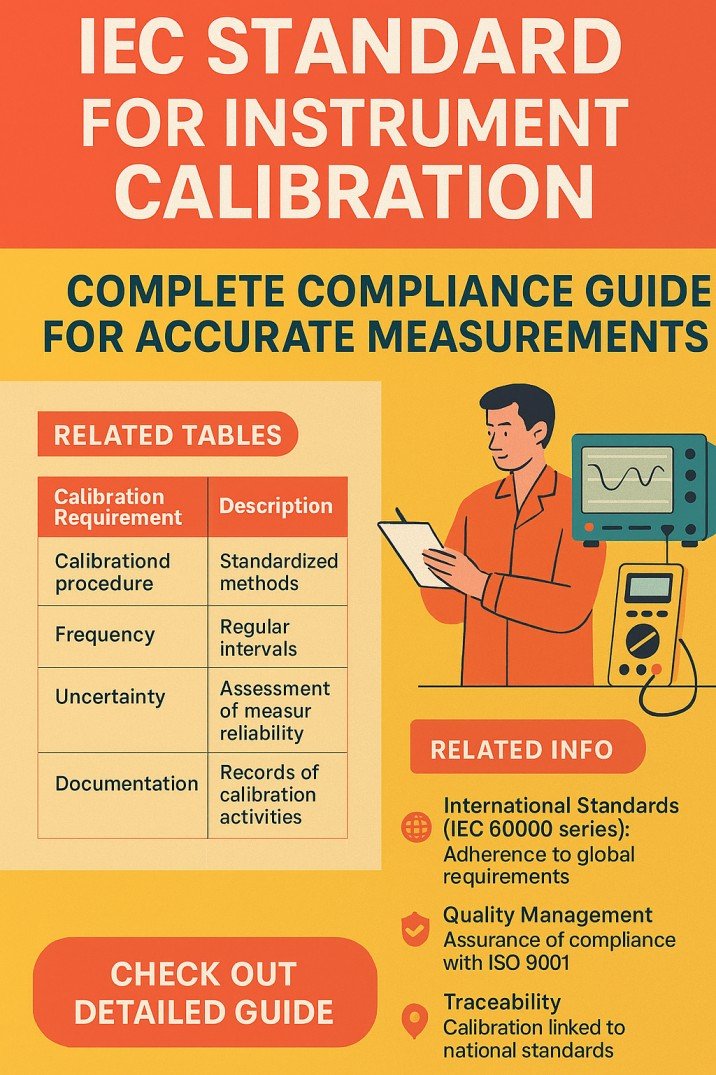
IEC Standard for Instrument Calibration: Complete Compliance Guide for Accurate Measurements
IEC Standard for Instrument Calibration explained in a practical way to ensure measurement accuracy, regulatory compliance, and reliable calibration practices in industrial and electrical systems.