Work Function of Metals: A Detailed Explanation
The work function of metals is a fundamental concept in physics and materials science, especially in fields like electronics, photoelectric effect, and semiconductor technology. It defines the minimum energy required to remove an electron from the surface of a metal. Understanding this property helps in designing electronic devices, solar cells, and vacuum tubes.
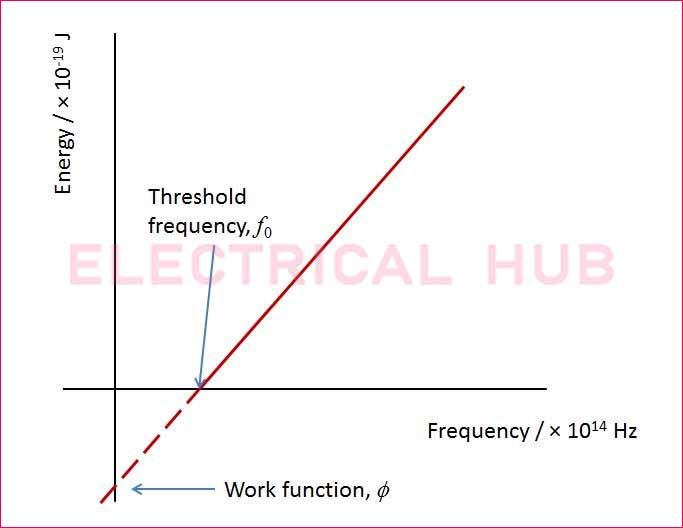
Table of Contents
Table of Contents
What is the Work Function of Metals?
The work function of metals is the minimum energy needed to extract an electron from the metal’s surface into free space. It is usually measured in electron volts (eV) and depends on the metal’s material properties, surface conditions, and temperature.
Factors Affecting Work Function
Material Composition – Different metals have different work functions due to variations in atomic structure.
Surface Conditions – Contaminants, oxidation, and surface roughness can alter the work function.
Temperature – An increase in temperature affects electron movement, slightly modifying the work function.
Crystal Orientation – The arrangement of atoms on the metal surface influences electron binding energy.
Work Function of Metals
| Metal | Work Function (eV) |
|---|---|
| Sodium (Na) | 2.75 |
| Aluminum (Al) | 4.28 |
| Copper (Cu) | 4.7 |
| Silver (Ag) | 4.26 |
| Gold (Au) | 5.1 |
| Iron (Fe) | 4.5 |
Work Function of Sodium
Sodium has a low work function of 2.75 eV, making it highly reactive and useful in photoelectric devices. Its low electron-binding energy allows easy emission of electrons when exposed to light.
Work Function of Aluminum
Aluminum has a work function of 4.28 eV and is widely used in semiconductors and coatings. It is less reactive than sodium but still plays a crucial role in electronic circuits.
Work Function and Threshold Frequency
The threshold frequency (f₀) is the minimum frequency of light required to eject an electron from a metal surface. This is related to the work function of metals using Einstein’s photoelectric equation:
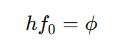
where:
- h is Planck’s constant (6.626 \times 10^{-34} , J·s)
- f0 is threshold frequency
- ϕ is the work function
Work Function and Threshold Frequency Relation
Since energy and frequency are related by E=hf, the work function of metals can be expressed as:
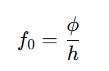
This means that metals with higher work functions require higher-frequency light to emit electrons.
Work Function and Threshold Frequency Formula
Using the above relation, the threshold frequency can be calculated by:
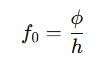
For example, for aluminum (ϕ=4.28 eV)
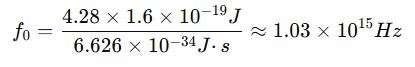
Work Function and Stopping Potential Relation
In the photoelectric effect, the stopping potential (Vs) is the voltage required to stop the ejected electrons from reaching the anode. It is related to the work function by:
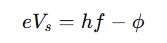
where ee is the electron charge (1.6×10−19C). This equation helps in determining the kinetic energy of emitted electrons.
Work Function in A-Level Physics
In A-Level physics, the work function is an essential topic under the photoelectric effect. It explains why certain metals eject electrons when exposed to light and how energy levels play a role in electronic transitions.
Work Function and Threshold Energy
Threshold energy is another term for the work function, representing the minimum energy needed for electron emission. It is crucial in solar cells, electron microscopes, and semiconductor devices.
Work Function and Electron Affinity
Electron affinity is the energy change when an atom gains an electron, while the work function is the energy required to remove an electron. Both concepts deal with electron interactions but in different ways.
Work Function and Fermi Level
The Fermi level is the highest energy level occupied by electrons at absolute zero temperature. The work function is the energy difference between the Fermi level and vacuum level. In metals, this value determines electrical conductivity and emission properties.
Work Function Dimensional Formula
The dimensional formula of work function is the same as energy:
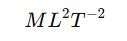
where:
- M represents mass
- L represents length
- T represents time
Work Function Definition Class 12
In Class 12 physics, the work function is defined as:
“The minimum energy required to remove an electron from a solid metal surface into free space.”
It plays a vital role in photoelectric effect experiments and semiconductor physics.
Work Function Definition in Physics
In physics, the work function is the energy necessary to move an electron from the Fermi level to vacuum level. It is a key parameter in electronic and optical devices.
Work Function Denoted By
The work function is denoted by ϕ or W in equations.
Work Function Dimensions
The dimensions of work function are the same as energy (Joules or eV), represented as .
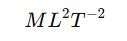
Work Function Depends on Frequency?
No, the work function is a fixed property of the metal. However, the energy of incident light must have a frequency above the threshold frequency to cause electron emission.
Work Function Definition in Photoelectric Effect
In the photoelectric effect, the work function is the energy barrier that must be overcome for electrons to be ejected when light falls on a metal. It follows Einstein’s equation:
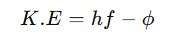
where K.E is the kinetic energy of emitted electrons.
Work Function in Chemistry
In chemistry, the work function is studied in relation to surface chemistry, catalysis, and electron emission. It helps in understanding chemical reactions on metal surfaces.
Work Function Activities
Some activities related to work function include:
- Measuring photoelectric emission using different metals
- Calculating stopping potential for different wavelengths
- Studying the effect of temperature on work function
Conclusion
The work function of metals is an essential concept in physics, electronics, and chemistry. It determines the ease with which electrons are emitted and influences various applications like solar cells, vacuum tubes, and semiconductors. Understanding the relationship between work function, threshold frequency, and stopping potential helps in developing advanced electronic systems.
For further details on work function, check out:
Follow Us on Social:
Subscribe our Newsletter on Electrical Insights for latest updates from Electrical Engineering Hub
#WorkFunction #Metals #PhotoelectricEffect #ElectronEmission #Physics #QuantumMechanics #SurfaceEnergy #MetalProperties #EnergyThreshold #MaterialScience #PhysicsConcepts #Electrons #VacuumEnergy #ScientificResearch #Engineering